Here are the sample questions which will help you be familiar with SAS Certified Clinical Trials Programming Using SAS 9 (A00-280) exam style and structure. We encourage you to try our Demo SAS Clinical Trials Programming Certification Practice Exam to measure your understanding of exam structure in an environment which simulates the SAS Certified Clinical Trials Programming Certification test environment.
Here are the sample questions which will help you be familiar with SAS Certified Clinical Trials Programming Using SAS 9 (A00-280) exam style and structure. We encourage you to try our Demo SAS Clinical Trials Programming Certification Practice Exam to measure your understanding of exam structure in an environment which simulates the SAS Certified Clinical Trials Programming Certification test environment.
To make your preparation more easy for SAS Certified Clinical Trials Programming (A00-280) exam, we strongly recommend you to use our Premium SAS Clinical Trials Programming Certification Practice Exam. According to our survey with certified candidates, you can easily score 85% in your actual SAS Certification exam if you can score 100% in our premium SAS Clinical Trials Programming Certification practice exams.
SAS A00-280 Sample Questions:
01. What is the main focus of Good Clinical Practices (GCP)?
a) harmonized data collection
b) standard analysis practices
c) protection of subjects
d) standard monitoring practices
02. The following SAS program is submitted:
proc sort data=SASUSER.VISIT out=PSORT;
by code descending date cost;
run;
Which statement is true regarding the submitted program?
a) The descending option applies to the variable CODE.
b) The variable CODE is sorted by ascending order.
c) The PSORT data set is stored in the SASUSER library.
d) The descending option applies to the DATE and COST variables.
03. Which statement correctly adds a label to the data set?
a) DATA two Label="Subjects having duplicate observations";
set one;
run;
b) DATA two;
Label="Subjects having duplicate observations";
set one;
run;
c) DATA two;
set one;
Label dataset="Subjects having duplicate observations";
run;
d) DATA two(Label="Subjects having duplicate observations") ;
set one;
run;
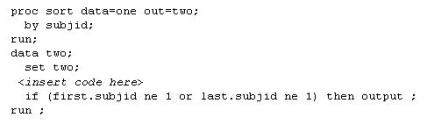
04. This question will ask you to provide a line of missing code. The following SAS program is submitted:
Which statement is required to produce this output?
a) TABLES site*group /nocol;
b) TABLES site*group /norow;
c) TABLES site*group;
d) TABLES site*group /nocol norow;
05. What information can be found in the SAS Dictionary tables?
There are two correct answer, Please select two correct answer.
a) datasets contained within a specified library
b) values contained within a specified format
c) variables contained within a specified dataset
d) values contained within a specified variable
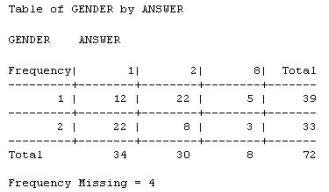
06. The following output is displayed:
Which SAS program created this output?
a) proc freq data=WORK.TESTDATA;
tables gender * answer / nocol norow nopercent;
run;
b) proc freq data=WORK.TESTDATA;
tables answer * gender / nocol norow nopercent;
run;
c) proc freq data=WORK.TESTDATA;
tables gender * answer / nocol norow nopercent missing;
run;
d) proc freq data=WORK.TESTDATA;
tables answer * gender / nocol norow nopercent missing;
run;
07. Which option in the PROC EXPORT procedure overwrites an existing file?
a) NEW
b) OVERWRITE
c) REPLACE
d) KEEP
08. Vital Signs are a component of which SDTM class?
a) Findings
b) Interventions
c) Events
d) Special Purpose
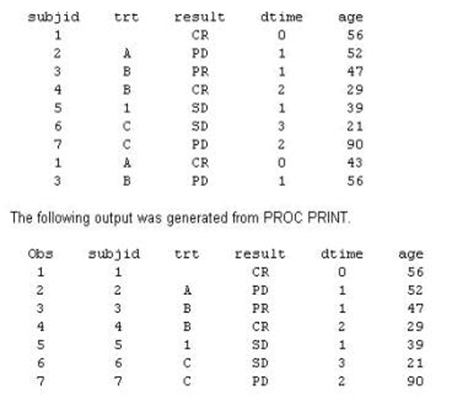
09. Given the following data set:
Which program was used to prepare the data for this PROC PRINT output?
a) proc sort data=one out=two;
by subjid;
run;
b) proc sort data=one out=two nodupkey;
by subjid;
run;
c) proc sort data=one out=two nodup;
by subjid;
run;
d) proc sort data=one out=two nodupkey;
by subjid trt;
run;
10. This question will ask you to provide a line of missing code. Given the data set WORK.STUDYDATA with the following variable list:
# Variable Type Len Label
2 DAY Char 8 Study Day
3 DIABP Num 8 Diastolic Blood Pressure
1 TRT Char 8 Treatment
The following SAS program is submitted:
proc means data=WORK.STUDYDATA noprint;
<insert code here>
class TRT DAY;
var DIABP;
output out=WORK.DIAOUT mean=meandp;
run;
WORK.DIAOUT should contain:
- the mean diastolic blood pressure values for every day by treatment group
- the overall mean diastolic blood pressure for each treatment group
Which statement correctly completes the program to meet these requirements?
a) id trt day;
b) by trt day;
c) where trt or trt*day;
d) types trt trt*day;
Answers:
|
Question: 1 |
Answer: c |
Question: 2 |
Answer: d |
|
Question: 3 |
Answer: d |
Question: 4 |
Answer: a |
|
Question: 5 |
Answer: a, c |
Question: 6 |
Answer: a |
|
Question: 7 |
Answer: c |
Question: 8 |
Answer: a |
|
Question: 9 |
Answer: b |
Question: 10 |
Answer: d |
Note: Please write us on feedback@analyticsexam.com if you find any data entry error in these SAS Certified Clinical Trials Programming (A00-280) sample questions.
 Here are the sample questions which will help you be familiar with SAS Certified Clinical Trials Programming Using SAS 9 (A00-280) exam style and structure. We encourage you to try our Demo SAS Clinical Trials Programming Certification Practice Exam to measure your understanding of exam structure in an environment which simulates the SAS Certified Clinical Trials Programming Certification test environment.
Here are the sample questions which will help you be familiar with SAS Certified Clinical Trials Programming Using SAS 9 (A00-280) exam style and structure. We encourage you to try our Demo SAS Clinical Trials Programming Certification Practice Exam to measure your understanding of exam structure in an environment which simulates the SAS Certified Clinical Trials Programming Certification test environment.